RESEARCH OVERVIEW
Research in the Wendt lab focuses on targeting molecular players involved in the metastatic growth of breast cancer. Systemic dissemination is an extremely early event in breast cancer progression, therefore we primarily focus on developing strategies that are specifically designed to target secondary tumors. We take two major approaches to thinking about this challenge:
1. Through metastatic progression breast cancer cells undergo epithelial-mesenchymal transition (EMT) and the reverse process of mesenchymal-epithelial transition (MET). An overriding hypothesis in the lab is that through the processes of EMT:MET the heterogeneity that characterizes the metastatic tumor is fundamentally unique from that of the primary tumor. We believe these differences hold the keys to developing therapeutics capable of treating metastatic lesions.
2. Subsequent to dissemination, a large fraction of tumor cells enter into an asymptomatic state of dormancy. The ability of normal organs to resist secondary tumor formation is the body’s last defense against metastatic disease progression. The environmental factors (drugs, alcohol, diet, exercise, etc…) involved in “awaking” of disseminated tumor cells are poorly characterized. Understanding of these factors is required if we hope to design therapeutics capable of maintaining systemic disease in an asymptomatic state of dormancy.
CURRENT PROJECTS
APPROACHES
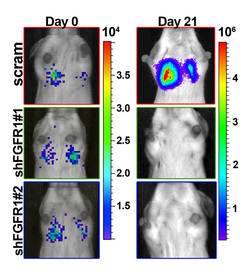
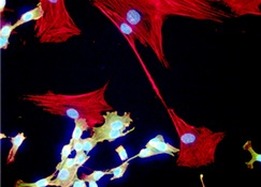
We use a combination of molecular, biochemical, cell biological and whole animal studies to evaluate the impact of anticancer therapies on EMT induction and metastatic progression. Specifically, we utilize a variety of 3D and multicellular colculture models to evaluate growth factor signaling, pharmacological response to anti-cancer drugs, and as a platform for genetic and compound screening assays. In addition to these in vitro approaches we have developed novel in vivo mouse models of metastasis and drug resistance. We utilize these models in combination with bioluminesent imaging as a robust approach to locate and quantify metastatic progression.
We use a combination of molecular, biochemical, cell biological and whole animal studies to evaluate the impact of anticancer therapies on EMT induction and metastatic progression. Specifically, we utilize a variety of 3D and multicellular colculture models to evaluate growth factor signaling, pharmacological response to anti-cancer drugs, and as a platform for genetic and compound screening assays. In addition to these in vitro approaches we have developed novel in vivo mouse models of metastasis and drug resistance. We utilize these models in combination with bioluminesent imaging as a robust approach to locate and quantify metastatic progression.